AG Grewal Announces Unsealing of Multi-State Complaint Alleging
Massive Generic Drug Price Fixing Conspiracy
Unredacted Emails Offer Look at Alleged Conspiracy to Inflate Prices, Nix Competition
TRENTON -- Attorney General Gurbir S. Grewal today joined 42 other states in releasing the full, unredacted complaint against some of the nation's largest generic drug manufacturers after a U.S. District Court Judge granted the states' motion to unseal the complaint. The complaint accuses 20 drug companies and 16 individual defendants – drug company executives responsible for sales, marketing, pricing and operations -- of conspiring to artificially inflate the prices of more than 100 generic drugs in violation of federal and state antitrust and consumer protection laws. More than half of the corporate defendants are based in New Jersey, and five of the individual defendants reside in the state. Contained in the unredacted complaint unsealed today are emails between generic drug manufacturers coordinating their response to a Congressional inquiry, emails enforcing "fair share" and "playing nice in the sandbox" market allocation, "fluff pricing" strategy and other coordination to artificially inflate prices, hinder competition and unreasonably restrain trade across the industry. “The information made public today paints a picture of some of our nation’s largest generic pharmaceutical companies conspiring to fix prices and allocate market shares for drugs that many people rely on to survive,” said Attorney General Grewal. “The defendants actively avoided written communications about their illegal conspiracy, but that did not stop our investigation from uncovering messages that open a window into their illegal actions to drive up drug prices for consumers in New Jersey and across the country.”
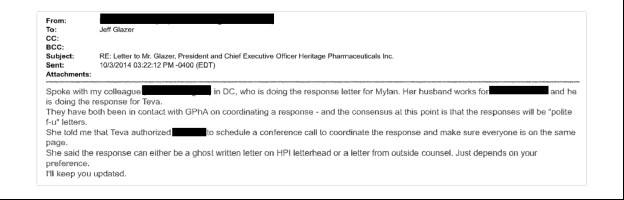
“Polite F-U Letters”
In early October 2014, Heritage Pharmaceuticals received a letter from Congressman Elijah Cummings and Senator Bernie Sanders as part of their joint investigation into price increases in the generic drug industry. Now emails unsealed by the court show that Heritage outside counsel immediately coordinated a response with counsel for Teva and Mylan.
“No emails please”
Executives knew their behavior was illegal, and they sought to cover their tracks by limiting communication in writing. Still, ample written evidence of their conspiracy has now been released that show consciousness of guilt.

(pg. 50)
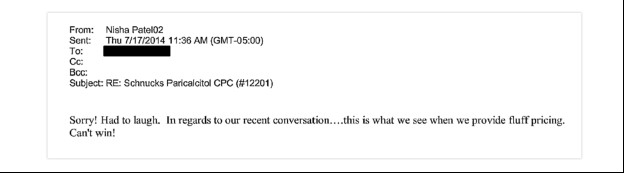
“I guess this is what they call co-opetition”
In one series of exchanges, senior executives at Mylan and Sandoz allegedly colluded to divvy up market share for a blood pressure medication. Both companies were the first to launch generic Valsartan on the same day, September 12, 2012. Leading up to the launch, records show company representatives spoke at least 21 times by phone to divvy up the market so that each competitor could obtain roughly 50 percent market share. The conspiracy apparently pleased company executives, including a Sandoz executive who states, "sometimes a little help from our competition is welcome as well." Another senior executive replied: "I guess this is what they call 'co-opetition."

High Quality Competitors
As Director of National Accounts at Teva, Defendant Nisha Patel's primary responsibility was to implement price increases. The complaint alleges that she did this by systematically conspiring with Teva's competitors and maintained a ranking system of Teva's competitors based on their collusive relationships, with +3 assigned to the most collusive and -3 assigned to the least. Detailed rankings and charts documenting her communication with competitors have been unsealed.
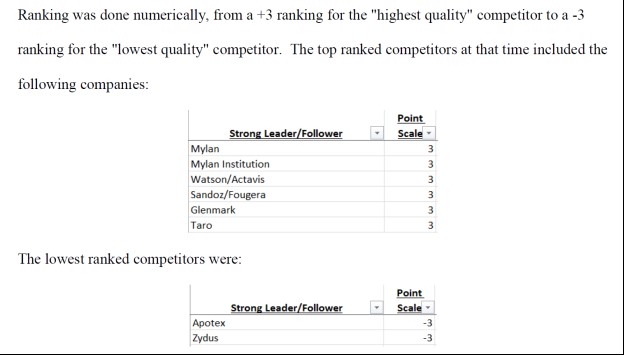 (pg. 176) (pg. 176)
 (pg. 183) (pg. 183)
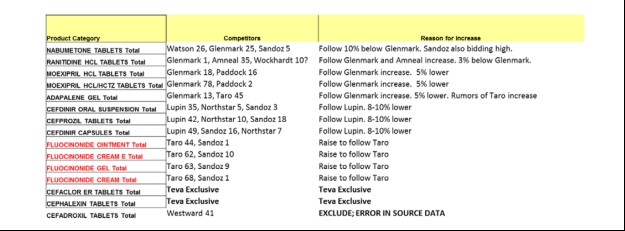
Industry Code Words Revealed: Playing Nice in the Sandbox, Fair Share, Fluff Pricing
The unredacted complaint reveals commonly used code words used by coconspirators as they colluded with competitors to divvy up market share and coordinate on price increases in violation of federal antitrust law. These include “fair share,” “playing nice in the sandbox,” and “fluff pricing.”


(pg. 147) Led by Connecticut, the multi-state lawsuit was filed May 10 in U.S. District Court for the District of Connecticut. The complaint is the second to be filed in an ongoing, expanding investigation that the Connecticut Office of the Attorney General has referred to as possibly the largest cartel case in the history of the United States. The first complaint, still pending in U.S. District Court in the Eastern District of Pennsylvania, was filed in 2016 and now includes 18 corporate defendants, two individual defendants, and 15 generic drugs. Two former executives from Heritage Pharmaceuticals, Jeffery Glazer and Jason Malek, have entered into settlement agreements and are cooperating with the Attorneys General working group in that case. Corporate defendants named in today’s lawsuit include the following. - Teva Pharmaceuticals USA, Inc., North Wales, PA
- Sandoz, Inc.,
Princeton, NJ
- Mylan Pharmaceuticals Inc.,
Canonsburg, PA
- Actavis Holdco US, Inc.,
Parsippany, NJ
- Actavis Pharma, Inc.,
Parsippany, NJ
- Amneal Pharmaceuticals, Inc.,
Bridgewater, NJ
- Apotex Corp.,
Weston, FL
- Aurobindo Pharma U.S.A., Inc.,
Dayton, NJ
- Breckenridge Pharmaceutical, Inc.,
Fairfield, NJ
- Dr. Reddy’s Laboratories, Inc.,
Princeton, NJ
- Glenmark Pharmaceuticals Inc. USA,
Mahwah, NJ
- Greenstone LLC,
North Peapack, NJ
- Lannett Company, Inc.,
Philadelphia, PA
- Lupin Pharmaceuticals, Inc.,
Baltimore, MD
- Par Pharmaceutical Companies, Inc.,
Chestnut Ridge, NY
- Pfizer, Inc.,
New York, NY
- Taro Pharmaceuticals USA, Inc.,
Hawthorne, NY
- Upsher-Smith Laboratories, LLC,
Maple Grove, MN
- Wockhardt USA, LLC,
Parsippany, NJ
- Zydus Pharmaceuticals (USA), Inc.,
North Pennington, NJ
### |
